Efficacy and Safety of Rapid-Acting Insulin Analogs in Special Populations with Type 1 Diabetes or Gestational Diabetes: Systematic Review and Meta-Analysis
- 230 Downloads
Abstract
Introduction
To assess the efficacy and safety of three available rapid-acting insulin analogs (insulins lispro, aspart and glulisine, respectively) in pregnant women, children/adolescents and people using continuous subcutaneous insulin infusion (CSII) with type 1 diabetes.
Methods
PubMed, EMBASE and Cochrane Reviews were searched electronically, and their bibliographies examined to identify suitable studies for review and inclusion in a meta-analysis. Eligible studies were randomized controlled trials that reported data on relevant clinical outcomes. A different reviewer abstracted data for each of the three subpopulations, and one reviewer abstracted data for all three. Any differences were resolved by consensus or by consulting a fourth reviewer.
Results
In people on CSII, rapid-acting insulin analogs lowered postprandial plasma glucose post-breakfast to a greater extent than did regular human insulin (RHI) (mean difference: − 1.63 mmol/L [95% confidence interval − 1.71; − 1.54]), with a comparable risk of hypoglycemia and a trend for lower glycated hemoglobin. In the pediatric population, glycemic control was similar with rapid-acting insulin analogs and RHI, with no safety concerns. Meta-analysis indicated severe hypoglycemic events were comparable for rapid-acting insulin analogs versus RHI (risk difference: 0.00 [95% confidence interval − 0.01; 0.01]). In the pregnancy group, insulin lispro and insulin aspart were safe and effective for both mother and fetus, with glycemic control being at least as good as with RHI. There were no data on insulin glulisine during pregnancy.
Conclusion
Rapid-acting insulin analogs appear generally safe and effective in these special populations; however, additional trials would be helpful.
Funding
Novo Nordisk A/S.
Keywords
CSII Pediatrics Pregnancy Rapid-acting insulin analogs Type 1 diabetesIntroduction
Many people with type 1 diabetes (T1D) receive insulin therapy [1]. Rapid-acting insulins are typically used to control postprandial plasma glucose (PPG) excursions, whereas long-acting basal insulins are used to control fasting glucose. Rapid-acting insulins are used as part of a basal–bolus injection regimen as well as for continuous subcutaneous insulin infusion (CSII). Three rapid-acting insulin analogs (RAIAs) are currently available in the USA and Europe: insulin lispro (Humalog®; Eli Lilly, Indianapolis, IN, USA), insulin aspart (Novolog® in the USA and NovoRapid® in the EU; Novo Nordisk, Bagsværd, Denmark) and insulin glulisine (Apidra®; Sanofi Aventis, Bridgewater, NJ, USA). All three of these RAIAs are also approved for the pediatric T1D patient population, although the ages on the product inserts for which there are data vary by product as well as by country (e.g. USA: insulin lispro, children ≥ 3 years of age; insulin aspart, ≥ 2 years; insulin glulisine, ≥ 4 years [2, 3, 4]; EU: insulin lispro, age not specified; insulin aspart, ≥ 1 years; insulin glulisine, ≥ 6 years) [5, 6, 7]. In addition, despite the concerns in some countries, the use of RAIAs is very high in T1D patients in general and is almost 100% among those patients using CSII [8].
The three insulin analogs lispro, aspart and glulisine differ in how their molecular structure has been modified from human insulin [9] and in the chemical composition of their formulations [10], but their pharmacokinetic (PK) and pharmacodynamic (PD) profiles are similar (Electronic Supplementary Material [ESM] Table S1). A large body of clinical studies indicates that these three RAIAs have similar efficacy and safety [11] and that they are preferred over regular human insulin (RHI) for use in adults with T1D due to their lower risk of hypoglycemia [12].
However, certain subgroups of patients with T1D (e.g. children and adolescents, pregnant women and people using CSII) are typically excluded from trials conducted for regulatory approval to ensure a more homogeneous group of patients.
A consequence of excluding these patients from regulatory trials is that there is some uncertainty about the clinical profile of RAIAs in patients who may have unique metabolic, developmental, cognitive or behavioral issues that materially affect the suitability of any medication. With RAIAs in widespread clinical use, it is timely to examine the available evidence for their performance in special populations. Thus, we performed a systematic review and meta-analysis of published data (PROSPERO registration #CRD42016043006).
Methods
Sources of Data and Search Criteria
The search terms “insulin lispro” (MeSH Terms) OR “insulin” (All Fields) AND “lispro” (All Fields) OR “insulin lispro” (All Fields) OR “lispro” (All Fields) OR aspart (All Fields) OR glulisine (All Fields) were used to search the PubMed, EMBASE and the Cochrane Reviews databases electronically on 1 June 2016 to identify records for further examination. The titles and abstracts (and, when necessary, full papers) were then screened to identify papers potentially reporting relevant in vivo data on safety or efficacy in randomized controlled trials (RCTs) involving one of three special populations (children and adolescents with T1D; pregnant women with pre-gestational T1D or gestational diabetes [GDM]; people with T1D using CSII). We did not set a date range and therefore included any study published up to the date of the search. Reference lists of retrieved publications and targeted review articles of RAIAs were also searched to identify additional records that might be provisionally relevant.
Selection of Studies and Eligibility Criteria
Records identified as provisionally relevant were then further examined for eligibility to verify that they were indeed RCTs, either blinded or open-label and of parallel or crossover design, in one of the target special populations and that they reported data on one of the identified clinical outcomes of interest. Eligibility for the three study populations was as follows: for the pregnancy population, women with either pre-gestational T1D or GDM; for the pediatric population, children or adolescents aged < 18 years with T1D; for the CSII population, people with T1D of any age using an insulin pump.
The criterion for the treatment in eligible studies was the administration of one of the three RAIAs compared with either RHI or each other; trials in which the effect of the RAIA could not be isolated (as in basal–bolus trials in which different basal insulins were used in each arm) were excluded. We did not pre-specify a minimum duration for the studies, although very short-term (e.g. 1- to 2-day meal tests designed to study PK/PD) were excluded. Outcome measures of interest included glycated hemoglobin (HbA1c), fasting blood glucose (FBG) or plasma glucose, PPG after any or all of the three main meals, hypoglycemia, hyperglycemia, diabetic ketoacidosis and/or pump/catheter occlusion and, for pregnancy, fetal outcomes.
Data Extraction
A standardized data abstraction form was adapted for use in this systematic review. A different reviewer abstracted data for each of the three subpopulations (KN, NS, SR) using the inclusion and exclusion criteria, and one reviewer abstracted data for all three of the subpopulations. Any differences were resolved by consensus or, if necessary, by consulting a fourth reviewer (PS) not involved in the original data abstraction process for that population. All reviewers are qualified at a post-doctorate level.
Data Synthesis and Statistical Analysis
For each of the three populations, whenever possible, we extracted data on glycemic control (HbA1c at baseline and end of trial; percentage of patients achieving HbA1c target[s] as specified in individual trials; 7- or 8-point self-measured blood glucose [SMBG]); and safety endpoints (e.g. severe, nocturnal, overall hypoglycemia; hyperglycemia/ketosis; fetal outcomes). For completeness, we also extracted other secondary endpoints if available. Each of the retrieved studies was assessed for study quality, including sample size, reporting of methods, reporting of results and risk of bias. Finally, using this information, we graded each study according to the following scale (−, +, ++), with − indicating poor quality, + indicating average quality and ++ indicating good quality.
All authors reviewed the available outcomes data for each eligible study and decided which studies had efficacy and/or safety data that would be suitable for data combination and meta-analysis within each of the three special populations. Suitability was determined by authors’ assessment of comparability of the outcomes assessed.
Meta-analysis was conducted using Review Manager 5 (RevMan 5.3 [http://community.cochrane.org/tools/review-production-tools/revman-5]) software for Cochrane Reviews. Two different outcome measures were used for the safety variables in the meta-analysis (namely risk difference for severe and any hypoglycemic episodes per month) and three outcome measures for the efficacy variables (namely mean difference in fasting and postprandial blood glucose [BG] and in HbA1c). All models were run as random-effects models. Statistical heterogeneity was calculated by using the I2 statistic, and publication bias was assessed by using a funnel plot and the Egger’s test.
Compliance with Ethics Statement
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Results
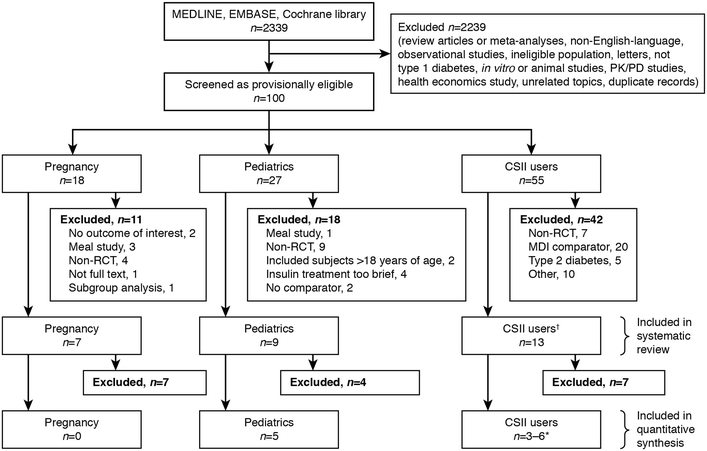
Flow diagram showing the number of retrieved, excluded and included records. The dagger (†) indicates that the continuous subcutaneous insulin infusion (CSII) group included two pediatric CSII studies. The asterisk (*) indicates that in the CSII meta-analyses, the number of studies included varied depending on the outcome assessed. MDI Multiple daily injections, PD pharmacodynamics, PK pharmacokinetics, RCT randomized controlled trial
Pregnancy
Study outcomes: pregnancy
| First author, year of study | Analog (n) vs. comparator (n) | HbA1c outcomes | BG levels | Hypoglycemia | Secondary outcomes | ||||
|---|---|---|---|---|---|---|---|---|---|
| Definition | Results | Definition | Results (mmol/L) | Definition | Results | Measures | Results | ||
| Aspart (157) vs. RHI (165) | Tested in early pregnancy and end of each trimester (1) Mean difference (95% CI) between aspart and RHI at end of second trimester (%) (2) Mean difference at end of third trimester | (1) − 0.04% (− 0.18; 0.11) (p = NS) (2) − 0.08 (− 0.23; 0.06) (p = NS) | 8-point SMBG performed for 1 week in each trimester (1) Mean 24-h BG at first trimester (2) Mean 24-h BG at second trimester (3) Mean 24-h BG at third trimester (4) Mean difference in post-breakfast glucose in aspart vs. RHI in first trimester (5) Mean difference in post-breakfast BG in second trimester (6) Mean difference in post-breakfast BG in third trimester | (1) 6.82 vs. 6.82 (2) 6.96 vs. 7.10 (3) 6.23 vs. 6.48 (4) NR, p = 0.044 (5) NR, p = 0.153 (6) NR, p = 0.0007 | Self-reported diary (1) Relative risk (95% CI) of major nocturnal hypoglycemia (2) Relative risk (95% CI) of major hypoglycemia in aspart vs. RHI | (1) 0.48 (0.20, 1.14) (p = NS) (2) 0.72 (0.36; 1.46) (p = NS) | (1) Mean (± SD) overall treatment satisfaction score (2) Corrected BW (SEM) (3) Preterm birth (4) Neonatal hypo | (1) 87.6 ± 12.0 vs. 83.4 ± 15.3 (p = 0.03) (2) 3438 (71.5) vs. 3555 (72.9) (p = 0.09) (3) 20.3% vs. 30.6% (p = 0.05) (4) 33.6 vs. 39.7% | |
| Persson, 2002 [15] | Lispro (16) vs. RHI (17) | Tested monthly (1) Median (range) HbA1c at baseline (6–8 weeks gestation) (%) (2) Median HbA1c at 24 weeks gestation (3) Median HbA1c before delivery | (1) 6.5 (4.8–8.6) vs. 6.6 (4.5–8.6) (2) 5.4 (4.3–5.9) vs. 5.3 (4.7–6.7), p = NS (3) 5.2 (4.6–5.9) vs. 5.0 (4.6–6.7), p = NS | Combined values in second and third trimester of 6-point SMBG (1) Mean (± SD) post-breakfast BG (mmol/L) (2) Mean post-breakfast glucose increase (3) Mean post-lunch glucose increase (4) Mean post-dinner glucose increase | (1) 6.50 ± 3.18 vs. 8.56 ± 3.55 (p < 0.01) (2) 0.40 ± 3.20 vs. 1.81 ± 3.42 (p < 0.01) (3) 0.70 ± 3.16 vs. 0.60 ± 3.34 (p = NS) (4) − 0.24 ± 3.10 vs. 0.28 ± 2.94 (p < 0.04) | Self-reported diary (1) Number of patients experiencing severe hypo (2) Rate of biochemical hypo (BG < 3.0) (%) | (1) 0 vs. 2 (2) 5.5 vs. 3.9 (p < 0.05) | (1) Progression of retinopathy, % in each group (2) Neonatal outcomes (i.e., anthropometry, complications, malformations, symptomatic hypo) | (1) 18.8 vs. 35.2 (2) No difference in perinatal outcome or neonatal complications |
| Mecacci, 2003 [17] | Lispro (25) vs. RHI (24) | Tested at diagnosis of GDM and delivery (1) Mean difference (± SD) from pre-intervention to delivery (%) | (1) − 0.3 ± 0.3 vs. − 0.3 ± 0.1 (p = NS) | 9-point SMBG performed weekly from diagnosis to 38 weeks gestation (1) Mean (± SD) 1-h post-breakfast BG (2) Mean BG 1-h post-lunch BG (3) Mean BG 1-h post-evening meal BG (4) Mean total BG 1-h postprandial BG (5) Mean total preprandial BG (6) Mean total 2-h postprandial BG | (1) post-BF: 5.93 ± 0.62 vs. 6.84 ± 0.7 (p < 0.002) (2) post-lunch: 5.91 ± 0.66 vs. 6.63 ± 0.88 (p < 0.01) (3) post-dinner: 6.21 ± 0.49 vs. 6.71 ± 0.62 (p < 0.05) (4) total: 6.02 ± 0.59 vs. 6.72 ± 0.73 (p < 0.01) (5) 4.08 ± 0.45 vs. 4.13 ± 0.73 (p = NS) (6) 5.20 ± 0.62 vs. 5.44 ± 0.69 (p = NS) | NR | NR | (1) Neonates with cranial-thoracic circumference (CC/CT) ratio 10-25th centile, % (b) Neonatal anthropometry (BW, ponderal index) and complications | (1) 12% vs. 37.5% (p < 0.05) (2) No difference between groups |
| Di Cianni, 2007 [16] | Aspart (31) vs. lispro (33) vs. RHI (32) | NR | NR | 5-point SMBG (1) Mean (± SD) 1-h post-breakfast BG | (1) 6.75 ± 1.12 vs. 6.6 ± 1.05 vs. 7.5 ± 1.3 (p < 0.05) | Number of patients experiencing any hypo | 0 vs. 0 vs. 0 | (1) Neonatal anthropometry: BW, CC/CT ratio (2) Macrosomia | (1) Higher BW in RHI than aspart/lispro groups (2) Macrosomia: 9.6%, 12.1%, 15.6% (p = NS) CC/CT ratio lower in RHI than other two groups (p = 0.03) |
| Pettitt, 2007 [18] | Aspart (14) vs. RHI (13) | Tested at diagnosis of GDM and delivery (1) Diagnosis (2) Delivery (both groups combined) | (1) 5.1 ± 0.4 vs. 5.3 ± 0.3 (2) 5.4 | Measured on standardized mixed meal test after 6 weeks (1) Time-adjusted mean (± SD) glucose (2) Time-adjusted mean change in glucose from baseline (no insulin) (3) Mean glucose at 30 min time point of meal test (4) Mean glucose at 60 min time point of meal test | (1) 4.2 ± 0.57 vs. 4.8 ± 0.86 (2) − 1.09 ± 0.55 vs. − 0.54 ± 0.74 (p = 0.003) (3) 4.7 ± 0.19 vs. 5.1 ± 0.23 (p = 0.03) (4) 5.4 ± 0.21 vs. 6.2 ± 0.33 (p < 0.01) | Self-reported diary (1) % of subjects (number of events) experiencing symptomatic hypo (2) % of subjects (number of events) experiencing minor hypo | (1) 71 (53) vs. 69 (23) (2) 79 (52) vs. 39 (9) | (1) Insulin-specific antibodies (2) Cross-reactive antibody binding (± SD) at 36/40 and 6/52 post-partum (%) (3) Mean neonatal birth weight (± SD) (kg) (4) Mean birth length (± SD) (cm) | (1) Low insulin-specific antibodies in both groups (2) 1.4 ± 3.0 vs. 1.5 ± 2.3 and 2.3 ± 5.4 vs. 6.5 + 13.7 (3) 3.1 (0.5) vs. 3.0 (0.5) (p = NS) (4) 49 (2.3) vs. 48 (2.4) (p = NS) |
| Jovanovic, 1999 [19] | Lispro (19) vs. RHI (23) | Tested at baseline and 6 weeks after randomization (1) Mean (± SEM) at baseline (%) (2) Mean at 6 weeks (3) Mean change from baseline to 6 weeks (% reduction) | Mean (± SEM) HbA1c at: (1) 5.47 ± 0.09 vs. 5.24 vs. 0.09 (p = NS) (2) 5.12 ± 0.11 vs. 5.16 ± 0.12 (3) − 0.35 (5.7%) vs. − 0.07 (2.8%) (p = 0.002) | 6-point SMBG (1) Mean (± SEM) post-breakfast hyperglycemia rate (individual patient % of all readings ≥ 6.67 mmol/L), (%) (2) Mean post-lunch hyperglycemia rate (3) Mean post-evening meal hyperglycemia rate (4) Mean total postprandial hyperglycemia rate | (1) 5.5 ± 0.30 vs. 7.3 ± 0.4 (p = NS) (2) 4.5 ± 1.1 vs. 6.8 ± 0.86 (p = NS) (3) 2.0 ± 0.51 vs. 2.6 ± 0.54 (p = NS) (4) 4.0 ± 0.49 vs. 5.5 ± 0.47 (p < 0.05) | Self-reported diary and meter check (1) Mean (± SEM) pre-breakfast hypo rate (individual patient % of all readings < 3.1 mmol/L) (%) (2) Mean pre-lunch hypo rate (3) Mean post-evening meal hypo rate (4) Mean total preprandial hypo rate | (1) 0.65 ± 0.13 vs. 0.93 ± 1.04 (p < 0.05) (2) 0.78 ± 0.37 vs. 1.98 ± 0.81 (3) 1.26 ± 0.43 vs. 1.43 ± 0.86 (4) 0.88 ± 0.25 vs. 2.20 ± 0.86 | (1) Change in insulin-specific and cross-reactive response from baseline to delivery (2) Neonatal anthropometry (3) Neonatal glucose | (1) No difference in maternal antibody response (2) No difference in neonatal anthropometry and outcomes (3) No neonatal hypo- or hyperglycemia |
Women with Pre-Existing T1D
Persson et al. compared treatment with insulin lispro with RHI treatment in a group of 33 pregnant women with T1D and found that the BG level was significantly lower after breakfast (but not after other meals) with insulin lispro (0.40 ± 3.20 vs. 1.81 ± 3.42 mmol/L; p < 0.01) [15] (Table 1). The 95% confidence interval (CI) was not significantly different between treatments, either at 24 weeks gestation or before delivery. The incidence of severe hypoglycemia was low (zero and two episodes for insulin lispro and RHI, respectively) and there were no differences in perinatal outcomes or neonatal complications.
The largest trial was an international, parallel-group trial enrolling 322 women with T1D who at enrollment were pregnant for ≤ 10 weeks or planning to become pregnant. Women were randomized to either insulin aspart or RHI, both in combination with neutral protamine Hagedorn insulin as the basal insulin [14]. HbA1c levels were comparable in the two groups at the end of the second and third trimesters (treatment difference, insulin aspart–RHI: − 0.04% [95% CI − 0.18; 0.11], − 0.4 mmol/mol [95% CI − 2.0; 1.2]; and − 0.08% [95% CI − 0.23; 0.06], − 0.9 mmol/mol [95% CI − 2.5; 0.7], respectively). Mean plasma glucose levels at 90 min post-breakfast were significantly lower in those women receiving insulin aspart arm than in those receiving RHI (p = 0.044 and p = 0.001 for end of first and third trimesters, respectively). The mean PPG increment across all meals was lower for the insulin aspart arm than for the RHI arm at the end of the first and third trimesters (estimated treatment difference: –0.75 [95% CI − 1.25; − 0.25], p = 0.003 and − 0.40 [95% CI − 0.80; − 0.01], p = 0.044, respectively). The risk of major hypoglycemic events was numerically lower, but not significantly different, for insulin aspart (rate ratio 0.72 [95% CI 0.36; 1.46]).
In a publication reporting additional data on pregnancy outcomes by Mathiesen et al. [14], Hod et al. [13] indicated that preterm delivery occurred in 20.3 and 30.6% of pregnancies in women receiving insulin aspart and RHI, respectively (p = 0.053). Other secondary publications from these trials indicated that there were 137 and 131 live births, 14 and 21 fetal losses, and six and nine congenital malformations in these groups of women on insulin aspart and RHI, respectively. Furthermore, maternal and cord blood antibody levels for both RHI and insulin aspart remained low for both treatments and were similar at 36 weeks gestation for the 97 women who participated in the substudy [20]. In a secondary analysis of data from the same trial, Lloyd et al. reported that these benefits were attained without increasing the cost of treatment compared to RHI [21].
Women with GDM
The characteristics of four RCTs using RAIAs in GDM are summarized in ESM Table S2 [16, 17, 18, 19]. The results indicate that insulin lispro was at least as effective as RHI and sometimes demonstrated improved glycemic control (ESM Table S2) [16, 17, 19]. Insulin aspart was associated with significantly lower post-meal BG compared with RHI [16, 18]. In the single trial involving a head-to-head comparison of insulin aspart and insulin lispro, mean 1-h post-breakfast BG was similar for the two products (6.75 ± 1.12 vs. 6.6 ± 1.05 mmol/L, respectively) [16].
In Mecacci et al. [17], hypoglycemia was not reported and, in another trial, there were no hypoglycemic events reported for insulin aspart, insulin lispro or RHI [16]. In a study of women (n = 27) using insulin aspart or RHI, the reported percentage of participants experiencing symptomatic hypoglycemic events was similar for both treatments (71 vs. 69%), but more participants using insulin aspart reported minor hypoglycemia (79 vs. 39%) [18]. In the latter case, this was largely due to two participants being prone to hypoglycemia. Neonatal outcomes (weight, length, physical exam) were good for both insulin aspart and RHI.
Meta-Analysis
The pregnancy studies were deemed to be too heterogeneous or to lack relevant information for meta-analysis of either efficacy or safety outcomes for any of the three RAIAs.
Children and Adolescents
Study outcomes: pediatrics
| First Author, year | Analog (n) vs. comparator (n) | HbA1c outcomes | BG levels | Hypoglycemia | Secondary outcomes | ||||
|---|---|---|---|---|---|---|---|---|---|
| Definition | Results | Definition | Results (mmol/L) | Definition | Results | Measures | Results | ||
| Philotheou, 2011 [29] | Glulisine (275) vs. lispro (295) | Tested at baseline and endpoint (1) Adjusted mean change (± SD) from baseline to endpoint (2) Difference between treatments in adjusted means (95% CI) for difference from baseline to endpoint (3) % achieving ADA age-specific HbA1c targets at endpoint | (1) + 0.10 ± 0.08 vs. + 0.16 ± 0.07 (2) − 0.06 (− 0.24; 0.12) (3) 38.4 vs. 32.0 (p = 0.0039) | 3-point SMBG at endpoint (1) Adjusted mean (± SEM) of pre-breakfast BG (p value for difference between groups) (2) Adjusted mean (± SEM) of pre-main meal BG (3) Adjusted mean (± SEM) of 2-h post main meal | (1) 8.77 ± 0.21 vs. 9.46 ± 0.21 (p = 0.014) (2) 9.76 ± 0.24 vs. 9.80 ± 0.23 (p = 0.894) (3) 9.20 ± 0.22 vs. 9.04 ± 0.21 (p = 0.564) | Reported from month 4 to treatment end (1) Severe hypo (± SD): BG < 2.0 mmol/L and third-party assistance required or prompt recovery following glucose treatment, number of episodes per patient-month (2) Nocturnal hypo (3) Any hypo | (1) 0.06 ± 0.24 vs. 0.07 ± 0.27 (2) 0.21 ± 0.50 vs. 0.20 ± 0.80 (3) 3.10 ± 4.33 vs. 2.91 ± 4.35 | Mean (± SD) increase total daily dose of insulin from baseline, units/day | 2.53 ± 0.68 vs. 4.91 ± 0.65 (p = 0.007) |
| Pańkowska, 2010 [28] | Aspart (20) vs. RHI (21) | Tested at baseline, midpoint and endpoint (1) Mean HbA1c (± SD) at 13 weeks after treatment (2) Mean HbA1c at 26 weeks after treatment | (1) 7.4 ± 0.9 vs. 7.6 ± 1.1 (2) 7.6 ± 0.9 vs. 7.6 ± 1.0 | 24-h glycemic control measured using CGMS for 72 h at endpoint (1) Mean (± SD) of area under glucose curve, mmol/h/L (2) Difference between maximum and minimum glucose levels over 24 h | (1) 219.8 (12.8) vs. 211.8 (10.9) (p = 0.55) (2) 4.0 vs. 4.0 (p = NS) | Self-reported by parent during CGM period (1) Severe hypo: BG < 2.8 mmol/L accompanied by CNS symptoms requiring external help, number of episodes per patient-year of exposure (2) Minor hypoglycemia: BG < 2.8 mmol/L that were asymptomatic or self-treatable (3) Symptoms-only hypoglycemia | (1) 0.1 vs. 0.0 (1/20) vs. (0/21) (2) 18 vs. 20 18/20 vs. 19/21 (3) 1.1 vs. 1.4 | Treatment satisfaction: SD values for mean change in treatment satisfaction score at end of study | 5.6 vs. 4.4 (p = 0.04) |
| Cherubini, 2006 [22] | Aspart (NR) vs. RHI (NR) | Tested at baseline and 6-weekly during study (1) Change in HbA1c (± SD) over the study period | (1) 7.5 ± 0.8 to 7.0 ± 0.4 vs. 7.5 ± 1.4 to 7.4 ± 0.5 (p = 0.018) | 7-point SMBG during study period (1) Mean 2-h postprandial BG (2) Mean fasting BG (3) Mean afternoon BG at endpoint (4) Decrease in mean daily BG variability from baseline | (1) 7.38 ± 1.0 vs. 7.94 ± 1.06 (p = 0.175) (2) Analog < RHI, p = 0.012 (3) 8.89 ± 2.89 vs. 9.17 ± 2.72 (p = 0.113) (4) Analog = RHI, p > 0.237 | Self-reported (1) Severe hypo: BG < 2.8 mmol/L, number of episodes per patient per day (2) Any hypo: BG < 3.9 mmol/L | (1) 0.045 vs. 0.035 (p = 0.209) (2) 0.214 vs. 0.18 (p = 0.117) | NR | NR |
| Fairchild, 2000 [25] | Lispro (35) vs. RHI (35) | Tested at baseline and 6-weekly during study (1) Mean HbA1c (± SD) at endpoint | (1) 8.33 ± 0.89 vs. 8.14 ± 0.77 (p = NS) | 7-point SMBG done weekly or 2-weekly (1) Mean BG (± SD) at 03:00 h (2) Mean difference (95% CI) in 03:00 h BG between groups | (1) 10.57 ± 0.26 vs. 9.02 ± 0.46 (p = 0.001) (2) 2.35 (−3.98; − 0.72) p = 0.01 | Self-reported and BG meter analysis (1) Severe hypos: hypo associated with convulsion or coma (2) Total recorded hypos, number of episodes per patient per 3 months (3) Total recorded hypos from 06:00 to 12:00 h (4) Hypos with BG < 3.0 mmol/L | (1) 0.032 vs. 0.065 (p = NS) 1/35 vs. 2/35 (2) 13.47 vs. 10.77 (p = NS) (3) 5.69 vs. 3.31, difference 2.4 ± 5.1 (p = 0.02) (4) analog = comparator (p = NS) | Treatment satisfaction questionnaire Number (%) preferring this type of insulin over the other one | 28 (80) vs. 7 (20) |
| Ford-Adams, 2003 [26] | Lispro (23) vs. RHI (23) | Tested at baseline and 4-monthly during study (1) Mean HbA1c (± SD) at crossover (2) Mean HbA1c at endpoint | (1) 8.9 ± 0.3 vs. 8.4 ± 0.3 (p = 0.14) (2) 8.5 ± 0.2 vs. 8.8 ± 0.3 (p = 0.47) | Overnight metabolic study (1) Mean BG (± SD) at start of overnight profile (2) Mean fasting BG (3) AUC of BG (± SD) from post-EM to bedtime, mmol/min/L (4) AUC of BG from 22:00 to 04:00 h | (1) 6.5 ± 1.0 vs. 7.1 ± 1.1 (p = 0.5) (2) 6.1 ± 0.8 vs. 6.3 ± 0.9 (p = 0.8) (3) 138 ± 12 vs. 170 ± 13 (p = 0.03) (4) 158 ± 13 vs. 145 ± 12 (p = 0.3) | Self-reported (1) Severe hypo: convulsions or requiring glucagon, number of episodes (2) Symptomatic hypoglycemia, number of episodes per patient per week (± SD) Overnight metabolic profile (3) Prevalence of low BG from post-EM to 22:00 h: BG < 3.5 mmol/L, % (4) Prevalence of low BG from 22:00 to 04:00 h (5) Prevalence of low BG from 04:00 to 07:00 h | (1) 2/23 vs. 1/23 (2) 1.6 ± 0.3 vs. 1.7 ± 0.3 (p = 0.2) (3) 9 vs. 6 (p = 0.06) (4) 8 vs. 13 (p = 0.01) (5) 27 vs. 22 (p = 0.11) | Mean (range) total daily insulin dose, units/kg | 0.97 (0.68–1.26) vs. 0.96 (0.53–1.22) (p = 0.2) |
| Holcombe, 2002 [27] | Lispro (457) vs. RHI (457) | Tested at baseline and 2-monthly during study (1) Mean HbA1c (± SD) at baseline (2) Mean HbA1c at endpoint | (1) 8.41 ± 1.4 vs. 8.80 ± 1.5 (p = NS) (2) 8.69 ± 1.52 vs. 8.70 ± 1.65 (p = NS) | 8-point BG profiles on 2 days at baseline and end of each treatment period (1) Mean (± SD) BG concentrations at 03:00 h (2) Mean fasting BG concentrations (3) Mean BG 2-h post-breakfast (4) Mean BG 2-h post-dinner (5) Mean BG 2-h post-mid-day meal | (1) 9.7 ± 3.9 vs. 8.8 ± 3.7 (p < 0.001) (2) 10.2 ± 3.5 vs. 9.6 ± 3.4 (p = 0.005) (3) 9.7 ± 4.0 vs. 10.6 ± 4.3 (p < 0.001) (4) 8.6 ± 3.5 vs. 9.3 ± 3.7 (p = 0.003) (5) 8.1 ± 3.4 vs. 8.5 ± 3.4 (p = NS) | Self-reported (1) Severe hypo: needing third-party assistance or intravenous glucose or glucagon injection, number of patients (2) Nocturnal hypoglycemia: from midnight to 06:00 h (3) Any hypoglycemia: symptoms present or measured BG < 3.0 mmol/L, number of episodes per patient per month | (1) 5 vs. 5 5/457 vs. 5/547 (2) 1.0 ± 1.9 vs. 1.7 ± 2.6 (p < 0.001) (3) 4.02 ± 4.5 vs. 4.37 ± 4.5 (p = 0.023) | (1) Mean (± SD) total daily insulin dose, units/kg (2) Mean (± SD) daily dose of short-and rapid-acting insulin | (1) 1.08 ± 0.32 vs. 1.05 ± 0.30 (p < 0.001) (2) 0.54 ± 0.24 vs. 0.53 ± 0.20 (p = NS) |
| Deeb, 2001 [24] | Lispro before meals (analog 1, 53) vs. lispro after meals (analog 2, 55) vs. RHI (57) | Tested at baseline and 3-monthly during study (1) Mean (± SD) HbA1c at endpoint | (1) 8.40 ± 1.1 vs. 8.54 ± 1.0 vs. 8.43 ± 1.0 (p = NS) | 7-point SMBG profiles on 2 days at baseline and end of each treatment period (1) Overall mean BG (± SD) (2) Mean BG 2-h post-breakfast (3) Mean BG pre-lunch (4) Mean BG 2-h post-lunch (5) Mean BG 2-h post-dinner BG | (1) 9.6 ± 1.6 vs. 10.1 ± 1.6 vs. 10.0 ± 1.7 (p = 0.007 analog 1 vs. analog 2 (p = 0.024 for trend) (2) 11.7 ± 4.4, 13.5 ± 5.5, 15.0 ± 5.4 (p < 0.001 analog 1 vs. comparator; p = 0.023 analog 1 vs. analog 2) (3) 8.7 ± 3.9 vs. 8.3 ± 3.1 vs. 8.3 ± 3.1 vs. 9.5 ± 4.1 (p = NS analog 1 vs. analog 2, p = 0.037 analog 1 vs. RHI) (4) Analog 1 = analog 2 = RHI (p = NS) (5) 8.8 ± 5.0 vs. 9.9 ± 4.7 vs. 10.8 ± 5.4 (p = NS analog 1 vs. analog 2, p = 0.006 analog 1 vs. RHI) | Self-reported (1) Severe hypo: needing third-party assistance, resulting in a coma, or requiring intravenous glucose or glucagon, number of patients (2) Any hypoglycemia: symptomatic or measured BG < 3.5 mmol/L, number of episodes per 30 days | (1) 2 vs. 3 vs. 6 (p = NS) 5/108 vs. 6/57 (2) 14.7 ± 11.9 vs. 13.6 ± 9.3 vs. 13.8 ± 9.8 (p = NS) | Mean total daily insulin dose, units/kg | 0.21 vs. 0.23 vs. 0.25 (p = NS) |
| Tupola, 2001 [30] | Lispro (22) vs. RHI (22) | Tested at baseline and 3-monthly during study (1) Mean (± SD) change in HbA1c from baseline to endpoint | (1) 0.2 ± 0.8 vs. − 0.4 ± 0.7 (p = 0.1) | 7-point SMBG profiles on 1 day per month over study period (1) Mean BG (± SD) pre-breakfast (2) Mean BG pre-dinner (3) Mean BG at bedtime (4) 1-h and 2-h postprandial BG excursions | (1) 11.5 ± 4.5 vs. 8.4 ± 3.8 (p = 0.02) (2) 11.7 ± 6.0 vs. 9.6 ± 5.7 (p = 0.4) (3) 11.5 ± 5.0 vs. 10.6 ± 6.0 (p = 0.8) (4) Analog = RHI (p = NS) | Self-reported and BG meter analysis (1) Severe hypo: loss of consciousness during a hypoglycemic episode (2) Nocturnal hypoglycemia: from 11:00 to 06:00 h (3) Hypoglycemia: symptoms present or BG < 3 mmol/L, number of episodes per patient per month | (1) 2 vs. 2 (p = NS) (2) 34 vs. 41 (p = 0.6) (3) 4.9 vs. 4.4 (p = 0.3) | (1) Total daily insulin dose (2) Patient satisfaction: number (%) of patients/families who wanted to continue this type of insulin | (1) Analog = RHI (p = NS) (2) 18 (82) vs. 4 (18) |
| Danne, 2007 [23] | Aspart (23) vs. RHI (25) | Tested at baseline and 3-monthly during study (1) HbA1c at endpoint | (1) Analog = RHI (p = NS) | 7-point SMBG during study period (1) Daily BG variations | (1) Analog = RHI (p = NS) | Self-reported (1) Major hypoglycemia: those that parents could not handle on their own, number of episodes (2) Any hypoglycemia, number of episodes per week (3) Relative risk of hypoglycemia (95% CI): relative risk for analog/RHI | (1) 2 vs. 3 (2) 2.75 vs. 2.74 (3) 1.06 (0.96;1.17) (p = 0.225) | WHO–DTSQs (1) Mean score (± SD) for question on continuing present form of insulin (2) Mean score (± SD) for question on recommending this form of insulin to others | (1) 4.8 ± 1.5 vs. 3.7 ± 1.8 (p = 0.045) (2) 5.0 ± 1.4 vs. 4.2 ± 1.8 (p = 0.051) |
Overall, glycemic control (either HbA1c or PPG) with insulin lispro or insulin aspart was equivalent to or better than that with RHI. This was also true with respect to incidence of hypoglycemic episodes or other adverse events. There were no head-to-head trials comparing all three RAIAs in pediatric participants. However, a large (n = 572), open-label, parallel-group, non-inferiority trial compared insulin glulisine with insulin lispro [29]. Insulin glulisine was demonstrated to be non-inferior to insulin lispro (treatment difference in HbA1c: − 0.06% [95% CI − 0.24; 0.12]; − 0.7 [95% CI − 2.6; 1.33] mmol/mol). More children achieved American Diabetes Association (ADA) age-specific HbA1c targets (at the time of the study: < 6 years, HbA1c > 7.5 to% < 8.5% [> 58 to < 69 mmol/mol]; 6–12 years, HbA1c < 8.0% [< 64 mmol/mol]; 13–17 years, HbA1c < 7.5% [< 58 mmol/mol]; currently, the ADA recommends HbA1c < 7.5% [< 58 mmol/mol] across all pediatric age groups [1]) with insulin glulisine than with insulin lispro (overall population: 38.4 vs. 32.0% for insulin glulisine and insulin lispro, respectively; p = 0.039). The frequencies of hypoglycemic events and other adverse events were similar.
Meta-Analysis
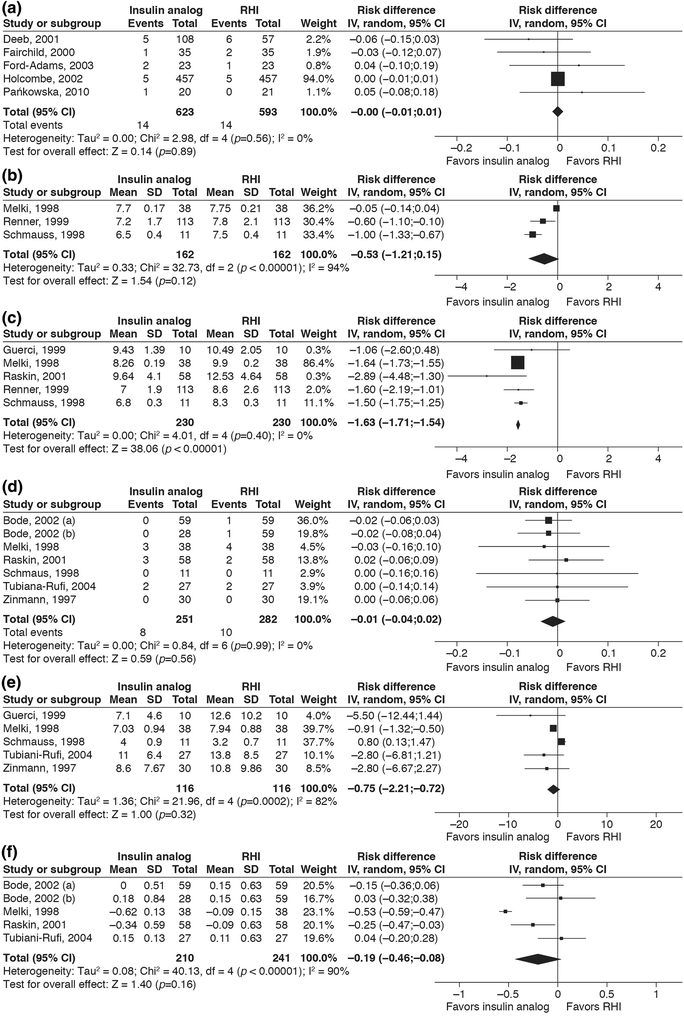
Meta-analyses of key outcomes. a Forest plot showing the difference in risk of severe hypoglycemic episodes with insulin analog treatment compared to regular human insulin (RHI) in a pediatric population. b Forest plot showing the difference in the mean fasting blood glucose level with insulin analog treatment compared to RHI treatment in the CSII sub-review. c Forest plot showing the difference in mean postprandial blood glucose (BG) level with insulin analog treatment compared to RHI treatment in the CSII sub-review. d Forest plot showing the difference in risk of severe hypoglycemic episodes with insulin analog treatment compared to RHI treatment in the CSII sub-review. e Forest plot showing the mean difference in the rate of any hypoglycemic episodes with insulin analog treatment compared to RHI treatment in the CSII sub-review. f Forest plot showing the difference in glycated hemogloblin (HbA1c) with insulin analog treatment (lispro or aspart) compared to RHI in the CSII sub-review.
Squares and diamonds represent the difference in HbA1c after intervention with the two treatments for each study (horizontal lines are 95% CI) and for all the studies combined, respectively. The I2 value refers to the statistical heterogeneity for the pooled analysis. A random-effects model using generic inverse variance showed a mean difference in HbA1c of − 0.19% (95% CI − 0.46; 0.08); − 2.1 (95% CI − 5.0; 0.9) mmol/mol with insulin analog compared to RHI at the end of the treatment period. The squares and the diamond in a, d, e represent the difference in risk for each study (horizontal lines represent 95% CI) and for all studies combined, respectively. The squares and the diamond in b, c represent the difference in the glucose levels between the two treatment arms for each study (horizontal lines are 95% CI) and for all the studies combined, respectively. The results of these meta-analyses are the mean of post-breakfast BG measurements only. In a and d ‘Events’ refers to the number of patients experiencing any such episode during the treatment period as a proportion of total number of patients in that treatment group. ‘Rate’ refers to mean (± SD) of any episodes of hypoglycemia per 30 days in all the patients in the respective treatment group. In f ‘Bode, 2002 (a)’ [31] refers to the observed difference in HbA1c between the subgroup of insulin lispro vs. RHI, and ‘Bode, 2002 (b)’ [31] refers to the subgroup on insulin aspart vs. RHI; the three remaining studies compare lispro vs. RHI. The ‘I2’ value refers to the statistical heterogeneity for this pooled analysis. CI confidence interval, IV inverse variance, SD standard deviation
Patients Treated with CSII
Study outcomes: continuous subcutaneous insulin infusion
| First author, year | Analog (n) vs. comparator (n) | HbA1c outcomes | BG levels | Hypoglycemia | Secondary outcomes | ||||
|---|---|---|---|---|---|---|---|---|---|
| Definition | Results | Definition | Results (mmol/L) | Definition | Results | Measures | Results | ||
| Zinmann, 1997 [41] | Lispro (30) vs. RHI (30) | Tested monthly (1) Mean (± SE) baseline HbA1c in all patients (2) Mean endpoint HbA1c (3) Difference in endpoint HbA1c between treatments | (1) 8.03 ± 0.13 (2) 7.66 ± 0.13 vs. 8.00 ± 0.16 (3) − 0.34 (p = 0.0041) | 8 point-SMBG profile done weekly (1) Mean fasting BG (2) Mean post-prandial BG | (1) No significant difference between treatments (2) Lower with lispro than with RHI (p < 0.05) | Self-reported diary (1) Any hypo at baseline: BG < 3 mmol/L or compatible symptoms, episodes per 30 days (± SE) (2) Any hypo during treatment (3) Biochemical hypo at baseline: BG < 3 mmol/L only (4) Biochemical hypo during treatment (5) Severe hypo | (1) 12.7 ± 1.6 (2) 8.6 ± 1.4 vs. 10.8 ± 1.8 (lispro p = 0.035 vs. baseline, RHI p = NS) (3) 8.4 ± 1.3 (4) 6.0 ± 0.9 vs. 7.6 ± 1.3 (lispro p = 0.03 vs. baseline, RHI p = NS) (5) 0/30 vs. 0/30 | (1) 5-h meal test on subgroup of six patients for postprandial insulin glucose and FFA (2) BW (3) Infusion-set occlusion | (1) Peak free plasma insulin at 45 min for lispro (287 ± 69 pmol/L) vs. 150 min for RHI (294 ± 56). Plasma glucose and FFA markedly reduced for lispro vs. RHI (2) No difference in BW from baseline or between treatments at endpoint (3) No occlusions with either treatment |
| Melki, 1998 [35] | Lispro (38) vs. RHI (38) | Tested at end of first 3 months (1) Baseline (2) Endpoint (3) Change from baseline to endpoint | (1) 7.74 ± 0.20 vs. 7.97 ± 0.13 (2) 7.11 ± 0.15 vs. 7.88 ± 0.16 (3) − 0.62 ± 0.13 vs. − 0.09 ± 0.15 (p = 0.01) | 7 point-SMBG profile done daily during last 30 days of each treatment period (1) Mean BG ± SE (2) Mean preprandial BG (3) Mean 2-h postprandial BG | (1) 7.93 ± 0.15 vs. 8.61 ± 0.18 (p < 0.0001) (2) 7.70 ± 0.17 vs. 7.75 ± 0.21 (p = NS) (3) 8.26 ± 0.19 vs. 9.90 ± 0.20 (p < 0.0001) | Self-reported diary recorded in last 30 days of first treatment period (1) Hypo event: BG < 3 mmol/L, episodes per month (± SD) (2) Very low BG measurement: BG < 2 mmol/L (3) Severe hypo: third-party assistance required | (1) 7.03 ± 0.94 vs. 7.94 ± 0.88 (p = NS) (2) 0.05 ± 0.05 vs. 0.47 ± 0.19 (p < 0.05) (3) 3 vs. 7 (3/38 vs. 4/38) | (1) Ketoacidosis (2) BW (3) Glucose variability (4) Patient preference (5) Technical problems | (1) No episodes in either group (2) No difference (3) Significantly lower overall mean and postprandial glycemic fluctuation with lispro than RHI, SD of BG in mmol/L (± SE): 3.44 ± 0.10 vs. 3.80 ± 0.10 (p < 0.001) and 3.58 ± 0.10 mmol/L vs. 3.84 ± 0.10 (p < 0.02) (4) All seven questions on non-validated questionnaire in favor of lispro (p < 0.0001) (5) Insulin precipitation in catheter, one vs. four episodes; catheter obstruction, nine episodes each |
| Schmauss, 1998 [38] | Lispro (11) vs. RHI (11) | Tested 3-monthly (1) Baseline (2) End of first 3 months (3) End of study | Lispro then RHI vs. RHI then lispro (1) 6.3 ± 0.2 vs. 6.7 ± 0.4 (p = NS) (2) 5.7 ± 0.3 vs. 6.5 ± 0.3 (p = NS) (3) 6.2 ± 0.2 vs. 6.3 ± 0.3 (p = NS) | 3-day BG profile done 3-monthly (1) Mean fasting BG (2) Mean 2-h postprandial BG | (1) 6.5 ± 0.4 vs. 7.5 ± 0.4 (p = NS) (2) 6.8 ± 0.3 vs. 8.3 ± 0.3 (p = 0.03) | Self-reported (1) Hypo: BG < 3.5 mmol/L and/or symptoms, episodes per 30 days (± SD) (2) Severe hypo: requiring IV glucose or glucagon | (1) 4.0 ± 0.9 vs. 3.2 ± 0.7 (p = NS) (2) 0 vs. 0 (0/11 vs. 0/11) | (1) Basal and bolus insulin requirements (2) BMI change (3) Treatment satisfaction | (1) No difference (2) No change in BMI (3) No difference in treatment satisfaction |
| Guerci, 1999 [32] | Lispro (10) vs. RHI (10) | Tested monthly (1) Baseline (2) Endpoint of each treatment period | (1) 7.17 ± 0.86 vs. 7.36 ± 0.76 (p = NS) (2) 7.07 ± 0.51 vs. 6.97 ± 0.67 (p = NS) | SMBG done monthly (1) Mean baseline BG (2) Mean BG at endpoint (3) Mean baseline postprandial BG (4) Mean postprandial BG at endpoint | (1) 9.35 ± 1.17 vs. 9.07 ± 0.43 (p = NS) (2) 9.04 ± 0.89 vs. 9.32 ± 1.17 (p = NS) (3) 9.53 ± 1.98 vs. 9.92 ± 1.05 (p = NS) (4) 9.43 ± 1.39 vs. 10.49 ± 2.05 (p = 0.05) | Self-reported (1) Incidence of hypo at baseline: BG < 3.5 mmol/L, episodes per 30 days (± SD) (2) Incidence of hypo at endpoint | (1) 10.1 ± 9.7 vs. 6.9 ± 4.4 (p = NS) (2) 7.1 ± 4.6 vs. 12.6 ± 10.2 (p = 0.05) | (1) Glucose variability, mean SD of previous months’ BG After pump interruption for 5 h: (2) Mean PG (3) Plasma 3-hydroxybuturate (4) Plasma FFA | (1) No difference between treatment groups (2) Until 3 h no difference in PG between groups. From 3 to 5 h, PG higher with lispro (p < 0.01) (3) From 3 h onwards, consistently higher for lispro vs. RHI (but p = NS) (4) From 2 h onwards, consistently higher for lispro vs. RHI (p < 0.05) |
| Renner, 1999 [37] | Lispro (113) vs. RHI (113) | Tested at baseline and endpoint (1) Baseline in all patients (2) Endpoint | (1) 7.24 ± 1.0 (2) 6.77 ± 0.88 vs. 6.90 ± 0.97 (p = 0.02) | 8-point daily SMBG (1) Mean fasting BG (± SD) (2) Mean post-breakfast BG (3) Mean pre-lunch BG (4) Mean post-lunch BG (5) Mean pre-dinner BG (6) Mean post-dinner BG (7) Mean 22:00 h BG (8) Mean 02:00 h BG | (1) 7.2 ± 1.7 vs. 7.8 ± 2.1 (p = NS) (2) 7.0 ± 1.9 vs. 8.6 ± 2.6 (p < 0.001) (3) 6.9 ± 1.9 vs. 7.3 ± 2.2 (p = NS) (4) 7.6 ± 1.9 vs. 8.7 ± 2.4 (p < 0.001) (5) 7.3 ± 1.9 vs. 7.5 ± 1.9 (p = NS) (p < 0.001) (6) 7.2 ± 1.9 vs. 8.3 ± 1.9 (7) 7.6 ± 1.8 vs. 8.3 ± 2.0 (p < 0.001) (8) 8.0 ± 2.7 vs. 7.7 ± 2.3 (p = NS) | Self-reported (1) BG < 3.5 mmol/L and/or symptoms, mean number of episodes (± SD) per patient per 4 months (2) BG < 3.3 mmol/L, mean number of episodes (± SD) per patient per month | (1) 12.0 ± 13.9 vs. 11.0 ± 11.2 (p = NS) (2) Lispro = RHI (p = NS) | (1) Ketosis (2) Treatment satisfaction (DTSQ) (3) Occlusion of catheter | (1) Five vs. four patients (2) Patients scored significantly higher on the treatment satisfaction when treated with lispro compared to RHI (3) Same amount in the two treatments |
| Johansson, 2000 [34] | Lispro (41) vs. RHI (41) | Tested at baseline and endpoint (1) Baseline in all patients (2) Endpoint (3) Difference between treatments (95% CI) | (1) 7.7 ± 0.8 (2) 7.4 vs. 7.6 (p = 0.047) (3) − 0.2 (− 0.3; 0.0) (p = 0.047) | Last 30 days SMBG (1) Mean of pre-prandial and bedtime BG (2) Mean of postprandial BG (3) Mean of all SMBG | (1) 8.5 vs. 8.4 (p = NS) (2) 8.1 vs. 9.6 (p < 0.001) (3) 8.3 vs. 8.9 (p < 0.001) | Self-reported (1) Any hypo: BG < 3.0 mmol/L and/or symptoms, episodes per 30 days (difference between treatments [95% CI]) | (1) 9.7 vs. 8.0 [1.7 (− 1.3; 5.3)] (p = NS) | (1) Treatment satisfaction (DTSQ) | (1) No difference between the treatments |
| Raskin, 2001 [36] | Lispro (58) vs. RHI (58) | Tested at baseline and endpoint (1) Baseline in all patients (2) Change from baseline | (1) 7.9 ± 1.1 vs. 7.6 ± 0.8 (p = NS) (2) − 0.34 ± 0.59 vs. − 0.09 ± 0.63 (p = 0.004) | 4-point daily SMBG done in last 2 weeks of each treatment period (1) Mean BG (± SD) PG during test meal at end of each treatment period (2) Mean PG 1 h post-meal (3) Mean PG 2 h post-meal | (1) 8.1 ± 2.0 vs. 8.1 ± 1.6, (p = NS) (2) 11.16 ± 4.29 vs. 13.20 ± 4.68 (p = 0.012) (3) 9.64 ± 4.10 vs. 12.53 ± 4.64 (p = 0.001) | Self-reported (1) Any hypo: BG < 3.0 mmol/L and/or symptoms, episodes per 12 weeks (2) Severe hypoglycemia: requiring IV glucose | (1) 8 vs. 11 (p = NS) (2) 3 vs. 3 (p = NS) 3/58 vs. 2/58 | (1) BW | (1) No difference between treatments |
| Tubiani-Rufi, 2004 [42] | Lispro (27) vs. RHI (27) | Tested at baseline and endpoint (1) Difference in HbA1c (± SD) from baseline to end of first treatment sequence | (1) + 0.15 ± 0.13 vs. + 0.11 ± 0.63 (p = NS) | 9-point SMBG on 2 days during each treatment (1) Change in mean post-dinner BG | (1) − 0.94 ± 5.05 vs. + 1.78 ± 5.94 (p = 0.01) | Self-reported (1) Severe hypoglycemia, episodes per 30 days (2) BG ≤ 3.3 mmol/L (3) BG ≤ 2.2 mmol/L | (1) 2 vs. 2 2/27 vs. 2/27 (2) 11.0 ± 6.4 vs. 13.8 ± 8.5 (p = NS) (3) 0.6 ± 1.1 vs. 1.0 ± 1.1 (p = NS) | (1) BW (2) Glucose variability (SD of SMBG) (3) Infusion-set occlusion (4) Parents’ treatment preference (5) Hyperglycemic episodes with ketonuria | (1) No difference between treatments (2) 6.4 ± 1.0 vs. 6.0 ± 0.95 mmol/L (p = 0.01) (3) No difference between treatments (4) 74% want to continue lispro (5) 2.5 ± 2.7 vs. 2.0 ± 3.0 episodes (p = NS) |
| Hoogma, 2006 [33] | Glulisine (29) vs. aspart (30) | Tested at baseline and endpoint (1) Baseline HbA1c (2) Endpoint HbA1c (3) Between-group difference in HbA1c (95% CI) from baseline to endpoint | (1) 6.8 vs. 7.1 (p = NS) (2) 7.0 vs. 7.2 (3) 0.11 (− 0.09; 0.31) (p = NS) | NR | NR | Self-reported (1) Severe hypoglycemia: BG < 2.0 mmol/L and requiring assistance or IV glucose/glucagon, number of patients experiencing at least one in 12 weeks (2) Nocturnal hypoglycemia (3) Symptomatic hypoglycemia | (1) 2 vs. 2 (2) 20 vs. 15 (p = NS) (3) 26 vs. 24 (p = NS) | (1) Infusion-set occlusion (2) Unexplained hyperglycemia: at least one episodes of BG > 19.4 mmol/L, episodes in 12 weeks | (1) No difference between treatments (2) 6 vs. 12 (p = NS) |
| Weinzimer, 2008 [43] | Aspart (198) vs. lispro (100) | Tested at baseline and endpoint (1) Baseline (2) Change in HbA1c from baseline to end of treatment (3) Percentage achieving age-specific HbA1c target at baseline (4) Percentage achieving age-specific HbA1c target at endpoint | (1) 8.0 ± 0.94 vs. 8.2 ± 0.84 (p = NS) (2) − 0.15 ± 0.05 vs. − 0.05 ± 0.07 (p = NS) (3) 50.3 vs. 40.4% (p = NS) (4) 59.7 vs. 43.8% (p = 0.04) | 8-point SMBG done on 2 days at baseline and before ending treatment (1) Mean fasting BG (± SD) at baseline (2) Mean fasting BG at endpoint (3) Mean postprandial BG at endpoint (4) Mean BG at endpoint | (1) 9.5 ± 4.3 vs. 9.9 ± 3.8 (p = NS) (2) 9.3 ± 3.7 vs. 10.0 ± 4.6 (p = NS) (3) aspart = lispro (p = NS) (4) aspart = lispro (p = NS) | Self-reported (1) Major hypoglycemia: BG < 3.1 mmol/L and requiring assistance or IV glucose/glucagon, episodes per patient per year (2) Nocturnal hypoglycemia: minor or major occurring between midnight and 06:00 h (3) Minor hypoglycemia: BG < 3.1 mmol/L with or without symptoms | (1) 0.4 vs. 0.3 (p = NS) (2) 5.7 vs. 6.2 (p = NS) (3) 77.2 vs. 66.0 (p = NS) | (1) BW (2) Hyperglycemia: BG > 16.7 mmol/L, % patients with episodes (3) DKA, number of patients with episodes | (1) No difference between treatments (2) 11 vs. 17 (p = NS) (3) 1 vs. 2 (p = NS) |
| Tamborlane, 2015 [39] | Substudy 1 lispro (116) vs. aspart (116) Substudy 2 lispro (118) vs. aspart (118) | Tested at baseline and endpoint (1) Mean (± SD) HbA1c at baseline (2) Mean (± SEM) endpoint HbA1c (3) Mean change (± SEM) in HbA1c from baseline | Substudy 1 (1) 7.3 ± 0.7 (2) 7.34 ± 0.05 vs. 7.28 ± 0.05 (p = NS) (3) 0.06 ± 0.05 vs. − 0.00 ± 0.05 (p = NS) Substudy 2 (1) 7.5 ± 0.7 (2) 7.30 ± 0.04 vs. 7.14 ± 0.04 (p < 0.001) (3) − 0.15 vs. − 0.31 (p < 0.001) | 7-point SMBG done on day 6 of reservoir use (1) Mean (± SEM) BG | Substudy 1 (1) 9.34 ± 0.14 vs. 9.11 ± 0.14 (p > 0.05) Substudy 2 (1) 8.72 ± 0.10 vs. 8.54 ± 0.10 (p > 0.05) | Self-reported (1) Severe hypo: requiring third-party assistance, total number of episodes (2) Nocturnal hypo: occurring between bedtime and wake up (3) Symptomatic hypo: BG < 3.9 mmol/L and symptomatic, episodes per 30 days (4) Symptomatic hypo: total number of episodes (5) Asymptomatic: BG < 3.9 mmol/L and no symptoms | (1) 5 vs. 16 (2) lispro = aspart (3) Substudy 1: 9.39 vs. 10.84 (p = 0.003) Substudy 2: 7.57 vs. 8.71 (p = 0.012) (4) Substudy 1: 15.26 vs. 16.91 (p = 0.006) Substudy 2: 16.74 vs. 18.86 (p < 0.001) (5) lispro = aspart | (1) Infusion-set occlusion (2) Hyperglycemia: BG > 13.9 mmol/L (> 3 h after eating) or > 16.7 mmol/L (within 3 h after eating), episodes per 30 days (3) DKA, number of episodes | (1) No difference between treatments (2) Substudy 1: 8.20 vs. 6.79 (p = 0.029) Substudy 2: 8.05 vs. 6.54 (p = 0.003) (3) 4 vs. 0 |
| Bode, 2002 [31] | Aspart (59) vs. lispro (28) vs. RHI (59) | Tested at baseline and endpoint (1) Mean (± SD) HbA1c at baseline (2) Mean change from baseline to endpoint | (1) 7.3 ± 0.7 vs. 7.3 ± 0.7 vs. 7.5 ± 0.8 (p = NS) (2) 0.00 ± 0.51 vs. 0.18 ± 0.84 vs. 0.15 ± 0.63 (p = NS) | 8-point SMBG done on 2 days at baseline and endpoint (1) Fasting and pre-meal BG (2) Post-dinner BG (3) BG at 02:00 h | (1) Aspart = lispro = RHI (2) Aspart < lispro = RHI (p = 0.019) (3) RHI < aspart = lispro (p = 0.002) | Self-reported (1) Severe hypo: BG < 2.8 mmol/L and requiring assistance or IV glucose/glucagon, mean events per patient per 30 days (± SD) (2) Nocturnal hypo: minor or major occurring between midnight and 06:00 h (3) Minor hypo: symptomatic and BG < 2.8 mmol/L (4) Minor hypo: symptoms with or without low BG | (1) 0 vs. 0 vs. 1 0/59 vs. 1/59; 0/28 vs. 1/59 (2) 0.5 ± 0.83 vs. 0.6 ± 0.61 vs. 0.9 ± 0.97 (aspart vs. lispro p = NS, aspart vs. RHI p = 0.004) (3) 3.7 ± 3.6 vs. 4.4 ± 4.7 vs. 4.8 ± 4.2 (p = NS) (4) 6.7 ± 5.4 vs. 10.5 ± 8.1 vs. 10.5 ± 8.9 (aspart vs. lispro p = 0.044, aspart vs. RHI p = 0.034) | (1) BW (2) Unexplained hyperglycemia: BG > 19.4 mmol/L, % patients with at least one episode (3) DKA | (1) No difference in any treatment group (2) 27% vs. 36% vs. 41%, (p = NS) (3) No episodes |
| van Bon, 2011 [40] | Glulisine (256) vs. aspart (256) vs. lispro (256) | Tested at baseline and endpoint (1) Mean (± SD) HbA1c at baseline (2) Mean HbA1c at endpoint (3) Percentage of patients with HbA1c < 7.0 at endpoint, % | (1) 7.31 ± 0.71 vs. 7.33 ± 0.71 vs. 7.28 ± 0.71 (p = NS) (2) 7.32 ± 0.73 vs. 7.26 ± 0.76 vs. 7.31 ± 0.74 (p = NS) (3) 28 vs. 31 vs. 30 (p = NS) | 7-point SMBG 1 day before endpoint (1) Fasting and pre-meal SMBG (2) Mean post-lunch BG (3) Mean nocturnal BG | (1) glulisine = aspart = RHI (2) (glulisine vs. aspart) 9.2 vs. 8.6 (p < 0.021) (3) (glulisine vs. lispro) 8.8 vs. 8.2 (p < 0.018) | Self-reported (1) Severe hypoglycemia: BG < 2.8 mmol/L and requiring assistance or IV glucose/glucagon, episodes per patient-year (± SD) (2) Nocturnal severe hypo: severe hypo occurring at night (3) Symptomatic hypo: BG < 3.9 mmol/L and symptomatic (4) Nocturnal symptomatic hypo: symptomatic hypo occurring at night | (1) 1.63 ± 4.50 vs. 1.38 ± 7.73 vs. 1.06 ± 3.81 (p = NS) (2) 0.61 ± 2.20 vs. 0.33 ± 1.18 vs. 0.36 ± 1.40 (glulisine vs. aspart p = 0.044, glulisine vs. lispro p = 0.070) (3) 73.84 ± 82.10 vs. 65.01 ± 72.07 vs. 62.69 ± 72.87 (glulisine vs. aspart p = 0.008, glulisine vs. lispro p < 0.001) (4) 12.80 ± 18.02 vs. 9.66 ± 13.75 vs. 9.48 ± 13.28 (glulisine vs. aspart p < 0.001, glulisine vs. lispro p < 0.001) | (1) Infusion-set occlusion (2) DKA (3) Hyperketonemia, rate per month (± SD) | (1) No difference between groups (2) 1 vs. 0 vs. 0 (3) 0.14 ± 0.43 vs. 0.06 ± 0.22 vs. 0.06 ± 0.18 (glulisine vs. aspart p = 0.01, glulisine vs. lispro p = 0.02) |
Eight studies compared an RAIA with RHI and all involved insulin lispro [32, 34, 35, 36, 37, 38, 41], with one being a pediatric trial [42]. All were crossover trials of 1–4 months’ duration. All indicated that insulin lispro was associated with improved glycemic control (HbA1c) and an incidence of hypoglycemic events that was similar to or lower than RHI.
Three studies involved head-to-head comparisons of insulin lispro versus insulin aspart [33, 39, 43], with an additional trial also comparing RHI [31]. The largest of the three trials was a 16-week, open-label RCT in 298 subjects with T1D aged 4–18 years [43] (ESM Table S6). At 16 weeks, the HbA1c in subjects receiving insulin aspart was deemed to be non-inferior to the HbA1c in those receiving insulin lispro, and there were no significant differences in FBG or rates of hyper- and hypoglycemia. However, the daily insulin dose was significantly lower for groups on insulin aspart (0.86 ± 0.237 vs. 0.94 ± 0.233 U/kg, for insulin aspart vs. insulin lispro, respectively; p = 0.018) [43]. In two related, 24-week, randomized, crossover trials in adults with T1D, insulin lispro was assessed to be non-inferior to insulin aspart based on SMBG profiles averaged over days 1–6 of treatment, but not when day 6 values alone were compared [39]. In a randomized, parallel-group trial in 146 adults with T1D, mean change from baseline was not significantly different for participants treated with insulin lispro, insulin aspart or RHI for 16 weeks [31]. Rates of hypoglycemia were also similar among treatments.
One RCT compared all three RAIAs in adults [40]. This was a crossover trial with three 13-week periods that was designed to test the superiority of insulin glulisine for unexplained hyperglycemia and/or infusion-set occlusion. It failed to show superiority of insulin glulisine on the primary outcome, but revealed that the monthly rate of unexplained hyperglycemic episodes and/or perceived catheter-set occlusion was significantly higher in insulin glulisine-treated patients than in those receiving the two other analogs [40]. Furthermore, insulin glulisine was associated with a higher frequency of symptomatic hypoglycemia, whereas HbA1c and 7-point SMBG were similar for all three insulin analogs [40].
Meta-analysis
Data on mean FBG and mean PPG for patients using CSII were sufficiently consistent to permit some meta-analysis, as were some of the hypoglycemic outcomes. Funnel plots suggested that there was no publication bias, although the number of studies in the meta-analysis with FBG outcomes was small (funnel plots not shown). Meta-analysis was performed for RAIAs versus RHI for FBG (three studies) (Fig. 2b), PPG (five studies) (Fig. 2c), severe hypoglycemic episodes (six studies) (Fig. 2d), any hypoglycemic episodes (five studies) (Fig. 2e) and HbA1c (four studies) (Fig. 2f). A random-effects model using generic inverse variance showed a mean difference in FBG of − 0.53 mmol/L (95% CI − 1.21; 0.15), a mean difference for PPG of – 1.63 mmol/L (95% CI − 1.71; –1.54), a risk difference for severe hypoglycemic episodes of − 0.01 (95% CI − 0.04; 0.02) and a mean difference in rate of any hypoglycemic episode of − 0.75 (95% CI − 2.21; 0.72). The mean difference in HbA1c was − 0.19% (95% CI − 0.46; 0.08); − 2.1 mmol/mol (95% CI − 5.0; 0.9) with RAIAs compared to RHI after 3 or 4 months of treatment.
Discussion
This systematic review and meta-analysis summarizes the safety and efficacy of RAIAs in populations of patients who are either typically excluded from clinical trials (i.e. due to pregnancy) or require dedicated trials (i.e. children and adolescents, patients using CSII). Overall, for insulin lispro and insulin aspart, data across all three special populations indicate that their safety and efficacy are comparable with, and in some cases significantly better than, RHI. Data also suggest, from the more limited results available, similar characteristics for insulin glulisine.
There are no head-to-head RCTs individually comparing all three RAIAs with each other in the pediatric CSII setting. However, in 2009, the Institute for Quality and Efficiency in Health Care in Germany, acknowledging the limited amount of data at the time, concluded that there were no significant differences between RHI and any of the three RAIAs in terms of key efficacy and safety endpoints [44]. The UK National Institute for Health and Care Excellence (NICE) guidelines specifically indicate that RAIAs are preferred over RHI for use in CSII for pediatric patients [45].
Given the lack of any major safety concerns when the three RAIAs were studied individually, there is no a priori reason to suspect that head-to-head trials would reveal any substantive differences in safety among them in special populations. However, without randomized comparative trials in these special populations, that conclusion remains speculative. Due to the limited number of studies and the heterogeneity of the outcome measures, only limited meta-analysis was possible, primarily in the CSII population. Those results indicate that RAIAs in CSII lower post-breakfast BG and possibly HbA1c to a greater extent than RHI, without an increased risk of hypoglycemia. Although many trials have been published using CSII versus multiple daily injections (MDI) in pediatric subjects, we did not review those here because it was impossible to separate the effects of the RAIA from those of the mode of treatment (CSII or MDI).
Conclusions
Rapid-acting insulin analogs appear to be safe and effective in these three special populations of people with T1D. However, additional trials would be helpful, and head-to-head trials would be necessary to detect any statistical differences among them, should they exist. The lack of clinically relevant differences in performance among the RAIAs make other factors, such as cost, availability and patient/provider preference, more important. Finally, studies have now been published indicating the PK advantages of a new formulation of insulin aspart in clinical development (faster aspart) over conventional insulin aspart [46], including in pediatric populations [47] and in those using CSII [48]. Studies addressing the performance of new fast-acting insulin aspart versus insulin glulisine and insulin lispro in these special populations in a clinical setting would be advantageous.
Notes
Acknowledgements
Funding
The authors received no funding for data abstraction, meta-analysis and writing. This work was supported by Novo Nordisk A/S. Novo Nordisk A/S funded the writing and editing assistance as well as the article publication charge, and reviewed the manuscript for scientific accuracy. The authors determined which studies were eligible for the review and meta-analysis, performed the meta-analysis and made the decision to submit the manuscript for publication.
Medical Writing and Editorial Assistance
The authors are grateful to Gary Patronek, Helen Marshall and Erin Slobodian, of Watermeadow Medical, an Ashfield company, part of UDG Healthcare plc, for writing and editing assistance in the development of this manuscript. Gary Patronek also performed the literature search and assisted with data abstraction. This assistance was funded by Novo Nordisk A/S, who also had a role in the review of the manuscript for scientific accuracy.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Kirsten Nørgaard serves as adviser to Medtronic, Abbott and Novo Nordisk, owns shares in Novo Nordisk, has received research grants from Roche and Novo Nordisk and has received fees for speaking from Medtronic, Roche, Rubin Medical, Sanofi, Novo Nordisk and Bayer. Ponnusamy Saravanan has received honoraria for serving on advisory boards and speaker fees for all three rapid-acting insulin analog-producing companies (Novo Nordisk, Sanofi and Lilly). Nithya Sukumar and Snorri B. Rafnsson declare they have no conflict of interest.
Compliance with Ethics Guidelines
This article is based on previously conducted studies, and does not involve any new studies of human or animal subjects performed by any of the authors.
Data Availability
Data used in the meta-analysis are included in Fig. 1.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Supplementary material
References
- 1.American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40[Suppl 1]:S1–135.Google Scholar
- 2.Eli Lilly. Humalog prescribing information. www.accessdata.fda.gov/drugsatfda_docs/label/2013/020563s115lbl.pdf; 2013. Accessed 29 Jan 2018.
- 3.Novo Nordisk. NovoLog prescribing information. www.accessdata.fda.gov/drugsatfda_docs/label/2015/020986s082lbl.pdf; 2015. Accessed 29 Jan 2018.
- 4.Sanofi Aventis. Apidra prescribing information. www.accessdata.fda.gov/drugsatfda_docs/label/2015/021629s030lbl.pdf; 2015. Accessed 29 Jan 2018.
- 5.Eli Lilly. Humalog product information. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000088/human_med_000820.jsp&mid=WC0b01ac058001d124; 2017. Accessed 29 Jan 2018.
- 6.Novo Nordisk. NovoRapid product information. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000258/human_med_000935.jsp&mid=WC0b01ac058001d124; 2017. Accessed 29 Jan 2018.
- 7.Sanofi Aventis. Apidra product information. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000557/human_med_000648.jsp&mid=WC0b01ac058001d124; 2017. Accessed 29 Jan 2018.
- 8.Bohn B, Karges B, Vogel C, et al. 20 years of pediatric benchmarking in Germany and Austria: age-dependent analysis of longitudinal follow-up in 63,967 children and adolescents with type 1 diabetes. PLoS One. 2016;11:e0160971.CrossRefPubMedPubMedCentralGoogle Scholar
- 9.Mooradian AD. Special considerations with insulin therapy in older adults with diabetes mellitus. Drugs Aging. 2011;28:429–38.CrossRefPubMedGoogle Scholar
- 10.Kerr D, Wizemann E, Senstius J, Zacho M, Ampudia-Blasco FJ. Stability and performance of rapid-acting insulin analogs used for continuous subcutaneous insulin infusion: a systematic review. J Diabetes Sci Technol. 2013;7:1595–606.CrossRefPubMedPubMedCentralGoogle Scholar
- 11.Home PD. The pharmacokinetics and pharmacodynamics of rapid-acting insulin analogues and their clinical consequences. Diabetes Obes Metab. 2012;14:780–8.CrossRefPubMedGoogle Scholar
- 12.Chiang JL, Kirkman MS, Laffel LM, Peters AL, Type 1 Diabetes Sourcebook Authors. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care. 2014;37:2034–54.CrossRefPubMedPubMedCentralGoogle Scholar
- 13.Hod M, Damm P, Kaaja R, et al. Fetal and perinatal outcomes in type 1 diabetes pregnancy: a randomized study comparing insulin aspart with human insulin in 322 subjects. Am J Obstet Gynecol. 2008;198(186):e1–7.Google Scholar
- 14.Mathiesen ER, Kinsley B, Amiel SA, et al. Maternal glycemic control and hypoglycemia in type 1 diabetic pregnancy: a randomized trial of insulin aspart versus human insulin in 322 pregnant women. Diabetes Care. 2007;30:771–6.CrossRefPubMedGoogle Scholar
- 15.Persson B, Swahn ML, Hjertberg R, et al. Insulin lispro therapy in pregnancies complicated by type 1 diabetes mellitus. Diabetes Res Clin Pract. 2002;58:115–21.CrossRefPubMedGoogle Scholar
- 16.Di Cianni G, Volpe L, Ghio A, et al. Maternal metabolic control and perinatal outcome in women with gestational diabetes mellitus treated with lispro or aspart insulin: comparison with regular insulin. Diabetes Care. 2007;30:e11.CrossRefPubMedGoogle Scholar
- 17.Mecacci F, Carignani L, Cioni R, et al. Maternal metabolic control and perinatal outcome in women with gestational diabetes treated with regular or lispro insulin: comparison with non-diabetic pregnant women. Eur J Obstet Gynecol Reprod Biol. 2003;111:19–24.CrossRefPubMedGoogle Scholar
- 18.Pettitt DJ, Ospina P, Howard C, Zisser H, Jovanovic L. Efficacy, safety and lack of immunogenicity of insulin aspart compared with regular human insulin for women with gestational diabetes mellitus. Diabet Med. 2007;24:1129–35.CrossRefPubMedPubMedCentralGoogle Scholar
- 19.Jovanovic L, Ilic S, Pettitt DJ, et al. Metabolic and immunologic effects of insulin lispro in gestational diabetes. Diabetes Care. 1999;22:1422–7.CrossRefPubMedGoogle Scholar
- 20.McCance DR, Damm P, Mathiesen ER, et al. Evaluation of insulin antibodies and placental transfer of insulin aspart in pregnant women with type 1 diabetes mellitus. Diabetologia. 2008;51:2141–3.CrossRefPubMedGoogle Scholar
- 21.Lloyd A, Townsend C, Munro V, Twena N, Nielsen S, Holman A. Cost-effectiveness of insulin aspart compared to human insulin in pregnant women with type 1 diabetes in the UK. Curr Med Res Opin. 2009;25:599–605.CrossRefPubMedGoogle Scholar
- 22.Cherubini V, Iannilli A, Iafusco D, et al. Premeal insulin treatment during basal-bolus regimen in young children with type 1 diabetes. Diabetes Care. 2006;29:2311–2.CrossRefPubMedGoogle Scholar
- 23.Danne T, Råstam J, Odendahl R, et al. Parental preference of prandial insulin aspart compared with preprandial human insulin in a basal-bolus scheme with NPH insulin in a 12-wk crossover study of preschool children with type 1 diabetes. Pediatr Diabetes. 2007;8:278–85.CrossRefPubMedGoogle Scholar
- 24.Deeb LC, Holcombe JH, Brunelle R, et al. Insulin lispro lowers postprandial glucose in prepubertal children with diabetes. Pediatrics. 2001;108:1175–9.CrossRefPubMedGoogle Scholar
- 25.Fairchild JM, Ambler GR, Genoud-Lawton CH, et al. Insulin lispro versus regular insulin in children with type 1 diabetes on twice daily insulin. Pediatr Diabetes. 2000;1:135–41.CrossRefPubMedGoogle Scholar
- 26.Ford-Adams ME, Murphy NP, Moore EJ, et al. Insulin lispro: a potential role in preventing nocturnal hypoglycaemia in young children with diabetes mellitus. Diabet Med. 2003;20:656–60.CrossRefPubMedGoogle Scholar
- 27.Holcombe JH, Zalani S, Arora VK, Mast CJ, Lispro in Adolescents Study Group. Comparison of insulin lispro with regular human insulin for the treatment of type 1 diabetes in adolescents. Clin Ther. 2002;24:629–38.CrossRefPubMedGoogle Scholar
- 28.Pańkowska E, Nazim J, Szalecki M, Urban M. Equal metabolic control but superior caregiver treatment satisfaction with insulin aspart in preschool children. Diabetes Technol Ther. 2010;12:413–8.CrossRefPubMedGoogle Scholar
- 29.Philotheou A, Arslanian S, Blatniczky L, Peterkova V, Souhami E, Danne T. Comparable efficacy and safety of insulin glulisine and insulin lispro when given as part of a basal-bolus insulin regimen in a 26-week trial in pediatric patients with type 1 diabetes. Diabetes Technol Ther. 2011;13:327–34.CrossRefPubMedPubMedCentralGoogle Scholar
- 30.Tupola S, Komulainen J, Jääskeläinen J, Sipilä I. Post-prandial insulin lispro vs. human regular insulin in prepubertal children with type 1 diabetes mellitus. Diabet Med. 2001;18:654–8.CrossRefPubMedGoogle Scholar
- 31.Bode B, Weinstein R, Bell D, et al. Comparison of insulin aspart with buffered regular insulin and insulin lispro in continuous subcutaneous insulin infusion: a randomized study in type 1 diabetes. Diabetes Care. 2002;25:439–44.CrossRefPubMedGoogle Scholar
- 32.Guerci B, Meyer L, Sallé A, et al. Comparison of metabolic deterioration between insulin analog and regular insulin after a 5-hour interruption of a continuous subcutaneous insulin infusion in type 1 diabetic patients. J Clin Endocrinol Metab. 1999;84:2673–8.PubMedGoogle Scholar
- 33.Hoogma RP, Schumicki D. Safety of insulin glulisine when given by continuous subcutaneous infusion using an external pump in patients with type 1 diabetes. Horm Metab Res. 2006;38:429–33.CrossRefPubMedGoogle Scholar
- 34.Johansson UB, Adamson UC, Lins PE, Wredling RA. Improved blood glucose variability, HbA1c insuman Infusat and less insulin requirement in IDDM patients using insulin lispro in CSII. The Swedish Multicenter Lispro Insulin Study. Diabetes Metab. 2000;26:192–6.PubMedGoogle Scholar
- 35.Melki V, Renard E, Lassmann-Vague V, et al. Improvement of HbA1c and blood glucose stability in IDDM patients treated with lispro insulin analog in external pumps. Diabetes Care. 1998;21:977–82.CrossRefPubMedGoogle Scholar
- 36.Raskin P, Holcombe JH, Tamborlane WV, et al. A comparison of insulin lispro and buffered regular human insulin administered via continuous subcutaneous insulin infusion pump. J Diabetes Complications. 2001;15:295–300.CrossRefPubMedGoogle Scholar
- 37.Renner R, Pfützner A, Trautmann M, Harzer O, Sauter K, Landgraf R. Use of insulin lispro in continuous subcutaneous insulin infusion treatment. Results of a multicenter trial. German Humalog-CSII Study Group. Diabetes Care. 1999;22:784–8.CrossRefPubMedGoogle Scholar
- 38.Schmauss S, König A, Landgraf R. Human insulin analogue [LYS(B28), PRO(B29)]: the ideal pump insulin? Diabet Med. 1998;15:247–9.CrossRefPubMedGoogle Scholar
- 39.Tamborlane WV, Renard E, Wadwa RP, et al. Glycemic control after 6 days of insulin pump reservoir use in type 1 diabetes: results of double-blind and open-label cross-over trials of insulin lispro and insulin aspart. J Diabetes. 2015;7:270–8.CrossRefPubMedGoogle Scholar
- 40.van Bon AC, Bode BW, Sert-Langeron C, DeVries JH, Charpentier G. Insulin glulisine compared to insulin aspart and to insulin lispro administered by continuous subcutaneous insulin infusion in patients with type 1 diabetes: a randomized controlled trial. Diabetes Technol Ther. 2011;13:607–14.CrossRefPubMedGoogle Scholar
- 41.Zinman B, Tildesley H, Chiasson JL, Tsui E, Strack T. Insulin lispro in CSII: results of a double-blind crossover study. Diabetes 1997;46:440–3. Erratum. Diabetes. 1997;46:1239.CrossRefGoogle Scholar
- 42.Tubiana-Rufi N, Coutant R, Bloch J, et al. Special management of insulin lispro in continuous subcutaneous insulin infusion in young diabetic children: a randomized cross-over study. Horm Res. 2004;62:265–71.PubMedGoogle Scholar
- 43.Weinzimer SA, Ternand C, Howard C, Chang CT, Becker DJ. Laffel LM; Insulin Aspart Pediatric Pump Study Group. A randomized trial comparing continuous subcutaneous insulin infusion of insulin aspart versus insulin lispro in children and adolescents with type 1 diabetes. Diabetes Care. 2008;31:210–5.CrossRefPubMedGoogle Scholar
- 44.Institute for Quality and Efficiency in Health Care: executive summaries. Rapid-acting insulin analogues in children and adolescents with diabetes mellitus type 1-follow-up commission: executive summary of final report A08-01, version 1.0. 2005–2009. Cologne, Germany: Institute for Quality and Efficiency in Health Care (IQWiG); 2009.Google Scholar
- 45.UK National Institute for Health and Care Excellence. Diabetes (type 1 and type 2) in children and young people: diagnosis and management. NICE guideline [NG18]. 2015. www.nice.org.uk/guidance/ng18 (Accessed 29 Jan 2018).
- 46.Heise T, Pieber TR, Danne T, Erichsen L, Haahr H. A pooled analysis of clinical pharmacology trials investigating the pharmacokinetic and pharmacodynamic characteristics of fast-acting insulin aspart in adults with type 1 diabetes. Clin Pharmacokinet. 2017;56:551–9.CrossRefPubMedPubMedCentralGoogle Scholar
- 47.Fath M, Danne T, Biester T, Erichsen L, Kordonouri O, Haahr H. Faster-acting insulin aspart provides faster onset and greater early exposure vs. insulin aspart in children and adolescents with type 1 diabetes mellitus. Pediatr Diabetes. 2017;18:903–10.CrossRefPubMedGoogle Scholar
- 48.Heise T, Zijlstra E, Nosek L, Rikte T, Haahr H. Pharmacological properties of faster-acting insulin aspart vs. insulin aspart in patients with type 1 diabetes receiving continuous subcutaneous insulin infusion: a randomized, double-blind, crossover trial. Diabetes Obes Metab. 2017;19:208–15.CrossRefPubMedGoogle Scholar